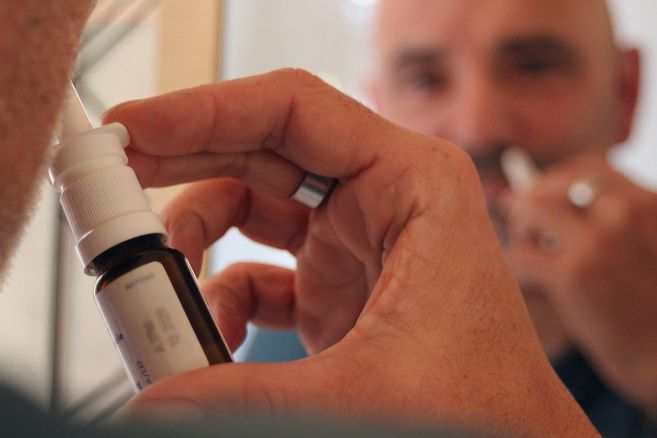
Masks, masks, hygiaphones, sprays, germicidal wipes, disinfectant drones … Over the past year, a large number of things have appeared in our daily life, with The stated goal to protect us from contamination by Covid-19.
The latest innovation to date:
- Un virucide nasal spray It is called “COV-Defense Nasal Spray” or “Biokami” based on ionized water, which will ensure that “Sars-CoV-2 is inactivated by more than 99%”, developed by Pharma & Beauty Group. (Editor’s note: other devices of the same type were developed throughout France “Covispray” from Vitrobio, in Issoire (Puy-de-Dôme), B Cell Design Spray, In Limoges …)
- Virus masks Which, too, will eliminate 99.9% of the virus “in thirty minutes” for “Viral Stop”, designed by Proneem, and “in five minutes” for a second, in the name of “Cidaltex”, produced by Bioserenity.
How did the nasal spray achieve these results? Are they credible?
99% efficiency, the argument is more than promising. But The National Agency for Medicines Safety (ANSM) warns: “Medical devices are considered one of the main health products, and therefore they are subject to strict control and follow-up by our departments, with the aim of monitoring compliance with the regulations applied to them, especially with regard to their performance and safety of use.”
Clearly: Only the Medical devices Following strict clinical protocols and testing A coordinator and a freelancer will receive a Marketing license. But at the moment, this is not the case with any nasal spray intended to fight Covid-19, ANSM notes: allegations that have been conveyed in recent days about Pharma & Beauty Spray in particular, neither from the product’s manufacturer and ANSM indicates that they “did not Receive any clinical data indicating the performance and safety of using this spray. ” Upon contact, Pharma & Beauty Group did not wish to respond to our questions “as long as discussions are ongoing with ANSM”.
Was it a marketing ploy or false hope?
The communication about the Pharma & Beauty nasal spray … was so viral! And it certainly fits with our desire to find a speedy solution to the crisis that affects us. So many media have transmitted information, and the existence of this product is now known to the largest number. However It cannot be marketed in France without a marketing authorization. The manufacturer might be disappointed to be able to launch its product on March 1.
But this isn’t necessarily false good news: ANSM has sent requests for additional information to the manufacturer So as to justify the alleged performance elements of its product “and to ensure that they do not pose any health risk. Once the file is created, ANSM will evaluate the product according to scientific criteria and then present it to the various committees, before giving an opinion. This could be favorable … as unfavorable) As for other sprays of this type, they are also under study.
What about antivirus masks?
The first, “Viral Stop” was designed and sold by Proneem. According to its manufacturer, “The efficacy of Viral Stop® tissue treatment has been demonstrated by A. Independent laboratory A French medical biotechnologist specializing in infectious diseases according to ISO 18184-2019 “ (Vibiosphen Lab, Editor’s Note); Was “introduced to Tests recommended by DGA“(Directorate General of Armament, Editor’s note), It was “Controlled by ANSES“(National Health Security Agency, Editor’s note) And “conforms to requirements AFNOR standard SPEC S76-001 manufacturer of barrier masks. “
The second, called “Cidaltex”, made by Bioserenity, was created thanks to Partnership between the University of Lille, Enserm (National Institute of Health and Medical Research, Editor’s note) And CNRS (National Center for Scientific Research, Editor’s note). Initially developed during the H1N1 influenza pandemic, it has been adapted “to meet the needs of the Covid-19 pandemic”. The manufacturer states that this technology “has been rigorously tested (…) by accredited laboratories” and that it meets “”EN-149 standard For “FFP2” quality masks.
Can we trust the indicators that manufacturers mention on their website?
According to Olivier Geibert, AFNOR Group Press Relation Officer (French Association for Standardization, Editor’s Note), The reference organizations “are interested in corporate advertisements in general” and ensure continuous monitoring. In the event of suspicion or reporting by consumers, Do not hesitate to call services scam (DGCCRF)Since the beginning of the health crisis. Consequently, companies marketing products that fight Covid-19 have every interest in citing organizations for which they have already gotten approval, at risk of prosecution.
What do health care professionals think?
For Dr. Thierry Prazuck, head of the infectious diseases department at Orléans Hospital, he announced the release of a nasal spray without previous study in vivo, that is, on real patients,This is not dangerousHe also questions the effectiveness of such a product as a preventive method: “The virus is not only in the nose, but also passes into the nasopharynx. There, the spray will do nothing. Then how long will it work? On an inert surface, it can work very well, but on Mucous membranes? Will it be necessary to apply every hour? Often? ‘ Unanswered questions in this time. “You have to do a full study, as you do with vaccines, otherwise it is worthless.”
Regarding anti-virus masks, Dr. Prazuck is not Not satisfied either : “For example, a DGA-verified mask, this does not mean that DGA interfered or gave its consent, but simply that the manufacturers followed one such or that action.” And to remember that between Various types of masks are available, FFP2 is already at least 94% aerosol and FFP3 is at least 99%. “ The main thing is to wear a mask, however [ces entreprises] NeedSelling point. Like washing powder that washes whiter! “
How do you make sure the new product presented as “Effective against Covid-19” is risk-free?
Olivier Gibert, of AFNOR, recommends that when faced with a new product, you always ask yourself: “Are the performance claims transparent? protocol Used? Are the tests performed by the company or by External lab ? Can I view the test reports? Is there a Standards International, European, French? This is the first sign of confidence. “
When in doubt, advises Olivier GeibertAsk your doctor as a priority, Or consumer associations like UFC-Que Choisir, or 60 million consumers. Because “the use of such a product is absolutely harmless, especially if it must be inhaled, swallowed, or applied to the skin.”
And to add that they are “just another tool” in the fight against Covid-19. “At the moment, in the area of prevention, it’s only the barrier signals that we all know have proven valuable.”
Maud Milikovic
– Focus on eight limousine companies, creative for a good reason
The health crisis accelerated innovations in Auvergne and Puy-de-Dôme
– Designed at the center, it is a “Citron Bracelet” aqueous alcohol gel dispenser
(Anti-viral), instructions for use. Every day, we will check data we observe on social media or in our discussions. We invite you to send us people of interest in your networks. We also invite you, within all articles, to let us know when you would like more details or additional information. Thank you for your loyalty.

“Web fanatic. Travel scholar. Certified music evangelist. Coffee expert. Unapologetic internet guru. Beer nerd.”